Sustained-Release Technology for Solid Preparation: SUSCRE Multi-Particulate Sustained-Release System
Technical core points: Based on the physiological characteristics and tolerance of children in different ages, the development of multi-particulate sustained-release preparation is under scientific design in consideration with the onset period of treatment for specific indications, based on the in vivo sustained-release pattern, and achieved through innovative formulation process technology.
Unique sustained-release mechanism: By simulating zero-order release model, pediatric drug administration can quickly reach and maintain a stable therapeutic plasma concentration, that is, by designing a combination of particles with different release rates to construct different release profiles, the immediate-release granules can quickly reach the onset concentration, while the sustained-release granules can be stably released within a set time to maintain the efficacy.
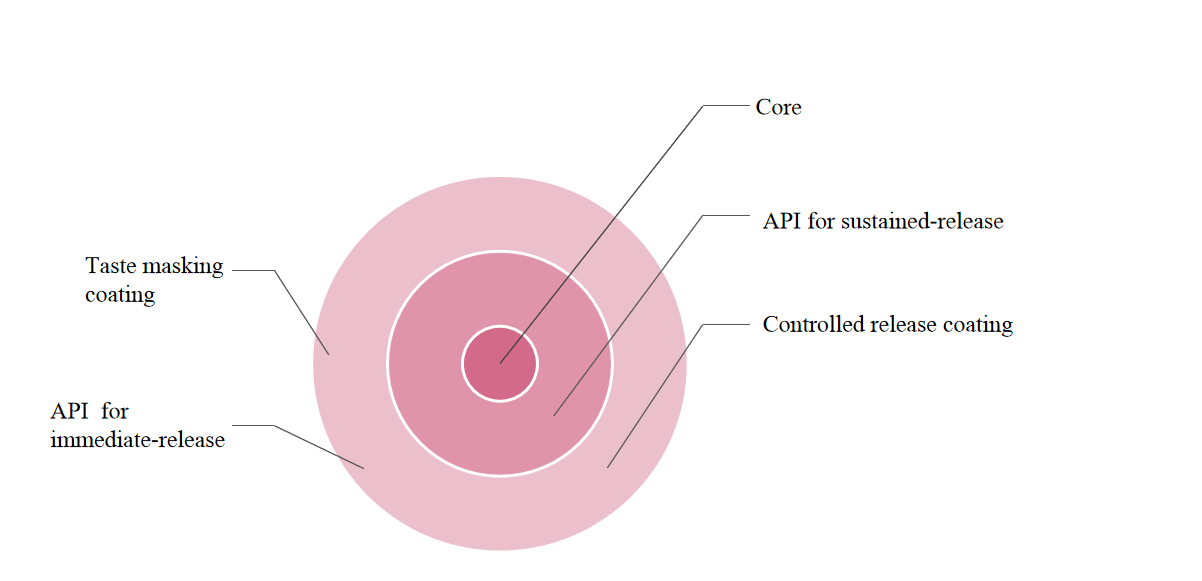
Clinical value focused: According to the physiological and psychological characteristics of each specific age group of children, the drug release technology is customized to achieve the drug release in a specific period and specific area by the designed release profiles, reducing drug doses, and improve the treatment compliance in children.
Taste-masking combined: Our pediatric drugs with two-layer coating realizes the combination of sustained-release technology and pediatric taste-masking to adjust the flavour for specific pediatric populations, enhancing the treatment compliance.
Sustained-Release Oral Suspension Technology: PENNKINETIE System
Technical breakthrough: Breakthrough the difficulty of the liquid system to ensure the sustained and controlled release effect, the sudden release of drugs in the aqueous environment is avoided, achieving the sustained release effect according to the set release rate;
Compound drug release mechanism: The innovative method of combining ion exchange technology and microparticle coating technology is used to form a unique resin microcapsule structure, which eliminates the impact of gastric emptying and prolongs the release time;
Good taste adjustment: The resin microcapsule uses the dual barrier of ionic complex and coating to effectively mask the bitter taste of the active ingredient, supplemented by Beimei's unique taste masking technology, to form a suitable taste for children and improve the medication compliance in pediatric population;
Convenient dosage adjustment: The liquid suspension system, equipped with special dosing devices, is designed to calculate the dosage according to the weight of the child population in kilograms, etc., to achieve accurate dosage adjustment and solve the shortcomings of pediatric clinical medication.