2021-10-28
奥维平®,whose generic name is Oseltamivir Phosphate for Oral Suspension, was formally approved by NMPA, the National Medical Products Administration recently.
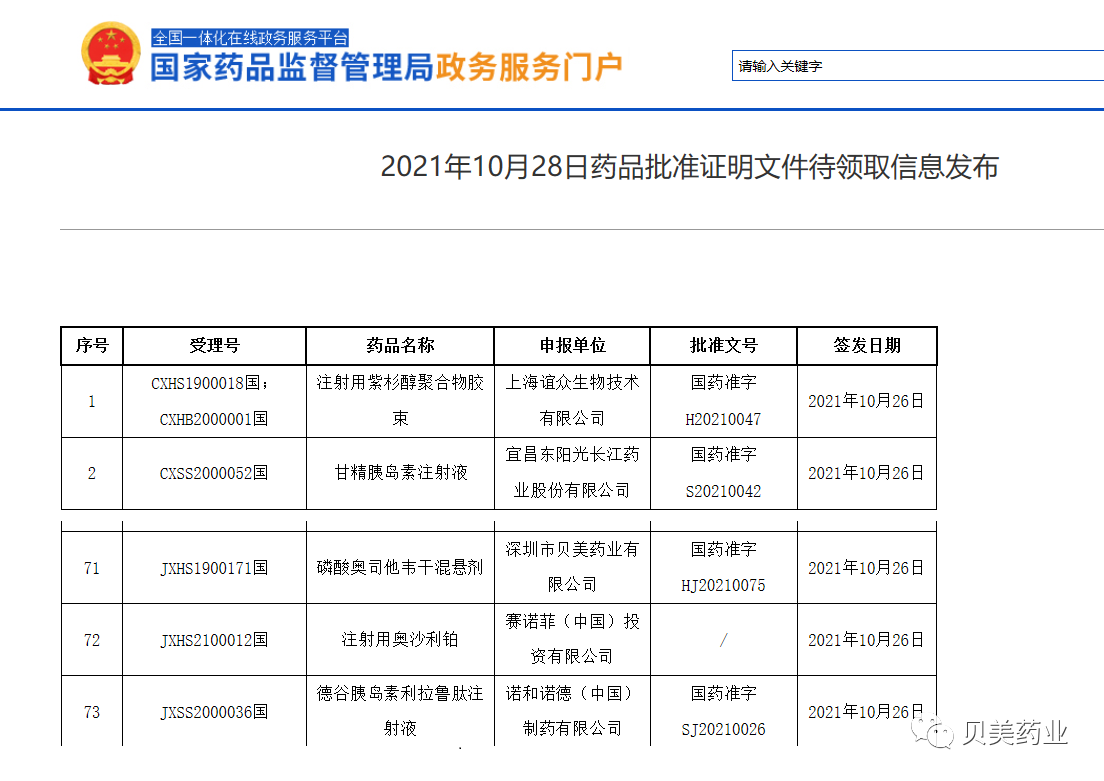
Oseltamivir Phosphate for Oral Suspension, as the first product for cooperation between Shenzhen Bemei Pharma and HETERO LABS LIMITED of India, its Regulatory application was officially accepted by the Center for Drug Evaluation, NMPA on December 12 in 2019, and formally approved on October 26 in 2021. This product is indicated for the treatment and prevention of acute, uncomplicated influenza A and B in children above 2 weeks of age and older.
Wu Guangmei,CEOof Beibei Pharma,said:"as the first approved Children's use dosage form of Oseltamivir China, Oseltamivir Phosphate for Oral Suspension has clear chinical value and advanages, such as precise drug delivery and good taste,which can target the clincal needs that the existing dosage forms cannot meet and benefit the majority of children and families."
The approval of 奥维平® (Oseltamivir Phosphate for Oral Suspension) will greatly enrich company product pipeline in the field of respiratory system.
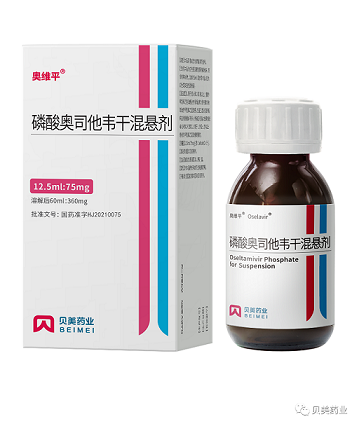
The news report by Heteron on Yahoo:
https://finance.yahoo.com/news/hetero-shenzhen-beimei-pharmaceuticals-launch-010000290.html?
guccounter=1&guce_referrer=aHR0cHM6Ly93d3cuZ29vZ2xlLmNvbS5oay8&guce_referrer_sig=
AQAAAD9CWSziEHxyrWmDBNQn4R5nGV5bJypuvfBT_y41I0xYpGM6POUyesYxNBYBShtJcMWAZSOljyvQRzf9_
wCWCEn7610QFSXI2Quske_aAS1MXkB_Ir9PLyimvLP5Hu2_v8tZuifmYv94WyWXd0ITNfTeHsOnoiEqTbe7mRMqIdfc