2021-11-17
Generic name: Atomoxetine Hydrochloride Oral Solution
Dosage form: Oral Solution
Registration category: Class 4
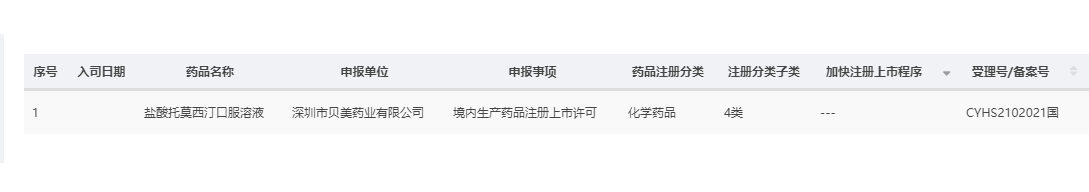
The first self-developed product of Beimei, Atomoxetine Hydrochloride Oral Solution, with its dossier completion of pharmacological studies and registration data, has been filed to the Center for Drug Evaluation (CDE) of the National Medical Products Administration on October 26, 2021, and the submission has been officially accepted by the CDE on November 12 (acceptance number CYHS2102021).
Atomoxetine Hydrochloride is a highly selective noradrenaline reuptake inhibitor that improves hyperactivity and attention deficit symptoms by increasing NE concentrations in the synaptic gap, but does not alter extracellular dopamine concentrations in subcortical and limbic nuclei regions, not inducing twitching or exacerbate dyskinesia, and is the first non-central-stimulant drug approved worldwide for the treatment of attention deficit/hyperactivity disorder (ADHD) in children and adolescents aged 6 years and older. With a high safety profile, no risk of abuse, and positive effects on co-morbidities, it is recommended both in the latest version of the American Academy of Pediatrics and Chinese ADHD guidelines as the first-line treatment for ADHD.
ADHD occurs mostly in childhood, and about half of the symptoms persist into adulthood. It is a group of syndromes characterized by marked difficulty in concentration, short attention span, hyperactivity or impulsivity. There are about 23 million children with ADHD in China, with a prevalence rate of 6.26%, but the hospital visit rate is only about 10%, and about 65% of the children have single or multiple co-morbidities, which seriously impairs the child's learning and social functions, and even involve other aspects of the whole life cycle. As families and the society pay increasing attention to childhood psychiatric disorders, the annual visit rate of ADHD has been enhanced in recent years, raising the demands for related therapeutic drugs.
According to reported statistics, the Chinese ADHD drug market is about 700 million RMB and is expanding at an average annual growth rate of over 30%. According to the overall terminal sales data from Minet.com, the sales of atomoxetine hydrochloride finished products in 2020 was 110 million RMB, with an average annual growth rate of 15%.
Clinically, when atomoxetine hydrochloride is used for treatment in children with ADHD, it is usually required to start with a dose of 40 mg or less, and then gradually increase the dose. At present, the most common capsule strengths in the market are 100 mg or above, with potential risks for pediatric drug use. In comparison, the oral solution form is more suitable for children, which can significantly improve the safety and compliance of medication, providing a better option for ADHD treatment.
Beimei’s first self-developed Atomoxetine Hydrochloride Oral Solution is provided with a set of dosing device to achieve accurate dosing and convenient administration, which is suitable for children. In addition, the product has a good taste and flavor that can be easily accepted by children, greatly increases the compliance of drug delivery. In the future, Beimei will constantly develop more products to file for launch.