2023-02-10
On February 03, 2023, Shenzhen Beimei Pharmaceutical Co., Ltd (hereinafter referred to as "Beimei Pharmaceutical") received the Acceptance Notice issued by the State Drug Administration of China (SDA), and the application for marketing authorization for the production of Desloratadine Oral Solution within the Company's territory was accepted.
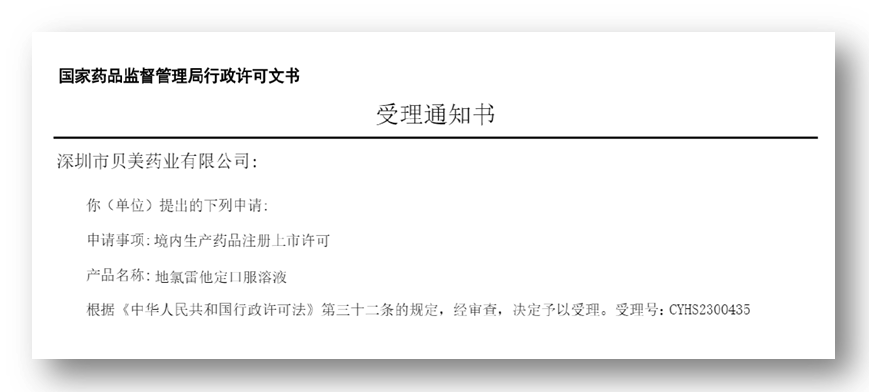
Desloratadine Oral Solution
This product is mainly applied to relieve the systemic and local symptoms of chronic idiopathic urticaria and allergic rhinitis. Chronic urticaria is a localized edema reaction due to dilation of small blood vessels and increased permeability of the skin and mucous membranes, which occurs daily or intermittently and lasts for more than 6 weeks. Allergic rhinitis is a symptom of rhinitis caused by the body's immune system coming into contact with external allergens. Desloratadine is one of the first choices for the treatment of the above-mentioned diseases. Due to the increase of sensitizing factors in the living environment, the number of patients with chronic urticaria and allergic rhinitis is increasing, and the distribution of the patients is gradually showing a younger trend.
Desloratadine oral solution offers the advantages of an oral solution dosage form, which is more suitable, compared with tablets, for the pediatric population and is expected to improve the medication compliance in children.
About Shenzhen Beimei Pharmaceutical Co., Ltd.

Beimei pharmaceutical focuses on the field of pediatric prescription medicines, headquartered in Shenzhen, China, incorporating R&D, global licensing, manufacture, marketing and distribution, and has more than 30 pediatric products in its portfolio and pipeline, covering the respiratory system, anti-infection (antibacterial), nervous system, digestive system, endocrinology, ENT and other fields.
Beimei Pharmaceutical has established long-term partnerships with companies such as Hetero, Cipla, EMP, Deva, Dr Reddy, LTS, MedPharma, Synthon, NTC, etc., and completed over 100 million RMB series A and series B/B+ financing, which will further support Beimei to be a pioneer in Chinese pediatric field to meet the clinically unmet demand and provide Chinese pediatric patients with products that are with high-quality, good tasting and can be accurately administrated.
For additional details and insights, kindly explore our official website: http://www.beimeiyaoye.com